American Canyon High School PE Teacher, Brad Rowell, Accused of 21 Felony Sex Crimes Against Student
Beware of Camp Lejeune Justice Act Fraud
Camp Lejeune Toxic Water Trials To Be Heard By Judge, Not a Jury
Recent Articles
$60 Million Verdict Could Lead to More NEC Baby Formula Lawsuits
An Illinois jury awarded $60 million last month to a woman whose premature baby died from necrotizing enterocolitis (NEC) after consuming baby formula. The jury found that Mead Johnson, the manufacturer of the popular formula brand Enfamil, acted negligently and failed to warn consumers about…
What Does a Federal Ban on Hair Relaxer Chemical Mean for Lawsuits?
In October 2023, it was revealed that the U.S. Food and Drug Administration (FDA) planned to propose a ban on chemical hair relaxers containing formaldehyde and formaldehyde-releasing chemicals. The proposal came just one year after yet another study found an association between ingredients in hair…
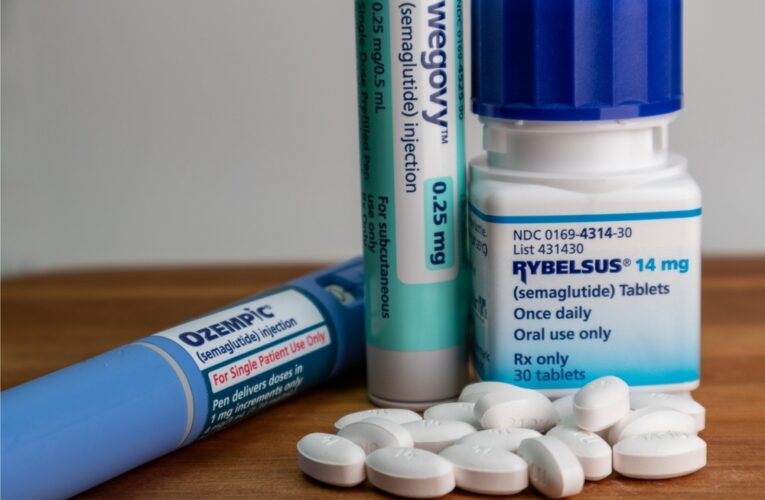
What Is Stomach Paralysis?
The number of people using diabetes weight loss drugs, prescriptions containing semaglutide, dulaglutide, liraglutide, or tirzepatide as a main ingredient, to lose weight has significantly increased in recent years. Unfortunately, some users have found that weight loss has come with a price – serious stomach…
Settlement Reached with Justice Department Following Massive CPAP Recall
Philips Respironics must stop producing sleep apnea devices in the U.S. until the manufacturer changes its safety measures per an agreement with the Department of Justice earlier this month. The government alleges that Philips violated the Federal Food, Drug, and Cosmetic Act (FDCA) during its…
FDA Issues Alert Over Heart Pump Deaths
A device meant to improve the outcomes of heart patients in life-threatening conditions is under fire from the FDA for its risk of puncturing the heart. Abiomed, a medical device manufacturer owned by Johnson & Johnson MedTech since 2022, is recalling instructions for using its…
What To Look for When Hiring a Roundup Lawyer
Lawsuits filed against Roundup manufacturer Monsanto and now Bayer have been filed across the country and are in various stages before the court system. Some lawsuits have been settled while others are waiting for their day in court. Roundup lawsuits claim that there is a…
Diabetes Weight Loss Drugs Lawsuits Consolidated
The request to consolidate lawsuits against pharmaceutical companies Novo Nordisk and Eli Lilly, who are under fire over the dangerous side effects linked to their explosively popular diabetes and weight loss drugs, was granted in February by the U.S. Judicial Panel on Multidistrict Litigation (JPML).…
Camp Lejeune Claims Portal Now Available
Many military veterans who have filed Camp Lejeune claims are still waiting for resolutions nearly two years after the Camp Lejeune Justice Act became law. A long-anticipated online portal is now available for Camp Lejeune claims, which could help expedite the process and finally bring…